[ad_1]
Within the Meals and Drug Administration’s (FDA) New Period of Smarter Meals Security, leaning into fashionable options to enhance meals security efforts is a serious focus. And whereas Part 204 of the Meals Security Modernization Act (FSMA 204) has been within the highlight this yr, they’re additionally bringing consideration to different key areas, together with modernizing recall occasions.
On September 29, 2023, the FDA held a public assembly with trade stakeholders throughout the US to share their feedback, considerations, and enter round modernizing recall methods for FDA-regulated merchandise. The assembly, titled “Modernizing FDA Recollects Listening Session,” featured feedback from two of Trustwell’s leaders: Chief Buyer Officer, Katy Jones, on behalf of Trustwell, and our Vice President of Provide Chain Methods and Insights, Julie McGill, on behalf of AIM North America.
Commenters had been requested to deal with a number of components, together with methods to cut back recall recurrence, making certain effectivity and effectiveness of recall methods, and methods for public warning, together with strategies to achieve underserved communities when remembers occur. The FDA will settle for written public feedback on the docket (FDA-2023-N-2393) till October 27, 2023. The four-hour recorded hybrid assembly occasion can be accessible to watch on the FDA’s web site.
FDA Remarks on Modernizing Recollects
Opening up the session, the FDA shared how necessary it’s to lean on the trade to raised perceive how rules and expertise can form meals security options and shield shoppers. Erik Mettler, Assistant Commissioner for Partnerships and Coverage, opened the assembly with these remarks:
“Throughout the board, we (the FDA and the federal authorities) need to perceive what actions are taking place on the bottom stage and the impression of these actions on the finish of the day. Recollects are an necessary a part of the availability chain and is the final line of protection for shoppers. What we actually need to do is determine how we are able to simplify the recall course of. The aim we’re right here in the present day is to take a look at the broad effort of recall processes within the trade… and the way we are able to transfer ahead with our recall actions.”
The assembly featured a panel of members throughout the FDA’s numerous places of work and departments, and trade stakeholders throughout the spectrum shared their feedback throughout the occasion to assist the FDA form future efforts on recall planning.
Fast Response is Important, However Requires Fashionable Options
Julie McGill shared her feedback because the 21st speaker (you may watch her portion on the 2:04:30 mark) on behalf of AIM North America because the co-chair of the Meals Provide Chain Work Group. AIM North America is a not-for-profit affiliation that permits cooperation, growth, and standardization of automated identification and knowledge seize (AIDC) expertise. These applied sciences embody barcodes, RFID, and ambient IoT, which have already begun to change how the trade traces merchandise throughout the availability chain and determine heaps for withdrawals.
Julie targeted her feedback on the expertise already used inside the trade and the way stakeholders can construct on this present basis.
“To see actual change, we acknowledge the significance of requirements, distinctive identification, knowledge sharing, and interoperability. AIDC applied sciences allow automation, improve accuracy, and improve efficiencies for recall operations. Modernizing remembers would require knowledge gathering throughout our provide chains, like what has been set within the Drug Provide Chain Securities Act, Distinctive Machine Identification, and FSMA 204.
 These rules play a pivotal function in provide chain visibility and traceability by offering a framework of actions and outlined knowledge attributes, and we encourage the FDA to take this into consideration as you start working in your recall modernization methods. Trade stakeholders, answer suppliers, trade teams, and different provide chain companions are utilizing terminology resembling ‘Crucial Monitoring Occasions’ and ‘Key Knowledge Parts’ of their companies and options in the present day. Let’s proceed to construct on this basis.
These rules play a pivotal function in provide chain visibility and traceability by offering a framework of actions and outlined knowledge attributes, and we encourage the FDA to take this into consideration as you start working in your recall modernization methods. Trade stakeholders, answer suppliers, trade teams, and different provide chain companions are utilizing terminology resembling ‘Crucial Monitoring Occasions’ and ‘Key Knowledge Parts’ of their companies and options in the present day. Let’s proceed to construct on this basis.
AIDC applied sciences allow customers to connect distinctive identifiers to merchandise, permitting for exact identification and isolation of affected gadgets throughout a withdrawal or recall. This not solely reduces the scope, but in addition minimizes disruptions to the availability chain and helps shield shoppers from probably dangerous merchandise. These options streamline communication between stakeholders by offering real-time knowledge sharing, and with enhanced visibility, customers have higher knowledge for recall notifications and updates. Modernizing recall expertise permits us to place that knowledge to make use of not solely in our operations, but in addition out to consumer-facing platforms, resembling cell apps, automated textual content messaging, and social media to inform shoppers rapidly and successfully.”
Closing out her feedback, Julie shared that the trade has entry to those AIDC instruments, however many within the trade have nonetheless uncared for to undertake fashionable instruments. And whereas change may be sluggish, not leaning into these recall options can finally result in dangerous or life-threatening occasions for shoppers. She ended her feedback with this: “By using these techniques and options, we’ve a chance to streamline operations, improve communications, and shield public well being. It’s time for trade to evolve and embrace recall modernization.”
A Rising Provide Chain Brings Challenges and Alternatives
Katy Jones was featured later within the lineup throughout the occasion (you may catch her remarks on the 4:19:55 mark). Katy’s remarks targeted on the intricate net of connections via the availability chain, and the complexity of information sharing throughout stakeholders. She additionally targeted on the significance of recent methods for notifying shoppers within the occasion of a recall, sharing:
“On this age of fast technological development, our technique of communication have developed dramatically. Nonetheless, our present recall system depends closely on conventional strategies, resembling press releases and paper notices. These outdated approaches can lead to vital delays in reaching shoppers who should be knowledgeable about probably harmful merchandise. By modernizing recall expertise, we are able to harness the facility of digital platforms, social media, texting, and cell apps to disseminate essential info quickly and effectively. This may assist notify shoppers, distributors, and retailers way more successfully, making certain that tainted merchandise are faraway from the market swiftly.”
Katy additionally targeted on the advantage of traceability expertise to reinforce gaps in recall response. Whereas FSMA 204 focuses totally on tracing merchandise throughout provide chains, lots of the instruments, language, and expertise being developed to satisfy compliance may also apply to the FDA’s recall processes.
 “Enhanced traceability, supported by the upcoming FSMA 204 necessities, means we are able to pinpoint the supply of contamination with precision and reduce the extent of remembers. This stage of traceability not solely aids in quicker remembers but in addition minimizes pointless waste by pinpointing the affected merchandise with accuracy.
“Enhanced traceability, supported by the upcoming FSMA 204 necessities, means we are able to pinpoint the supply of contamination with precision and reduce the extent of remembers. This stage of traceability not solely aids in quicker remembers but in addition minimizes pointless waste by pinpointing the affected merchandise with accuracy.
Moreover, modernizing recall expertise is not only about reacting to contamination incidents; it is also about prevention. By using knowledge analytics and synthetic intelligence backed by sturdy vitamin evaluation and high quality administration techniques, we are able to proactively determine potential dangers within the meals provide chain. This permits us to take preventive measures earlier than a recall turns into essential. This shift in direction of predictive analytics can save lives and shield public well being.”
In her place as Chief Buyer Officer, Katy can be carefully acquainted with the impression Trustwell’s expertise has had on recall efforts. She shared with the panel that Trustwell’s FoodLogiQ Recall has helped clients cut back the time to execute a withdrawal by over 70%. In different phrases, what as soon as took days now solely takes clients a couple of hours to finish – making certain a extra fast response that may shield client well being.
Katy ended her feedback with a private message:
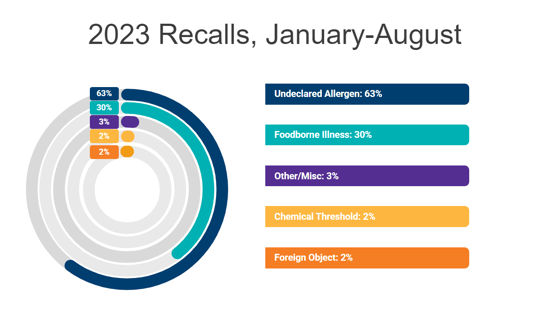
“Lastly, I converse in the present day not simply as an advocate for meals expertise however as a mother. Undeclared allergens made up the largest proportion of US remembers total within the final 18 months, making up 47% of all remembers in 2022 and 63% of remembers in 2023, thus far. And for my son, who suffers from a life-threatening tree nut allergy, the velocity through which meals corporations are in a position to talk and totally resolve a recall as a consequence of an undeclared allergen is paramount.”
Simply as our meals provide chain has developed quickly up to now few years, so should our response system to remembers. Katy, Julie, and lots of others on the FDA’s public assembly shared the significance of harnessing expertise, and the challenges that may be solved via superior, tech-enabled options.
Constructing a Higher, Safer Future for Recollects
The FDA is constant to just accept feedback on this measure till October 27, 2023. When you’ve got considerations, questions, or feedback that you simply want to share with the FDA, you are able to do so on the Federal Register web page for this docket. As soon as the FDA has closed the remark interval, they’ll start compiling info on how they’ll form future recall insurance policies and rules.
At Trustwell, we perceive the significance of fast recall response and defending client well being is carefully tied to our mission of adjusting the meals trade for the higher. To be taught extra about our FoodLogiQ Recall answer, or any of our suite of meals security, labeling, and compliance merchandise, attain out in the present day to schedule a customized demo with our group.
[ad_2]
Source link









